Nanowires of gallium phosphide can increase the production of hydrogen in solar panels
One of the most advanced forms of artificial photosynthesis is to use solar energy for the cleavage of liquid water into oxygen and hydrogen, which can be used as a clean fuel. The most effective semiconductor material for this is gallium phosphide (GaP), with which you can convert sunlight into electricity, as well as to share the water. Unfortunately, this stuff is too expensive for mass production, but the researchers were able to use a gallium phosphide in processed form, to create a prototype of a solar cell, which requires 10 000 times less than that of the material, but it is 10 times increases the production of hydrogen.
GaP has the potential to create solar fuel cell “all in one”, and researchers from Eindhoven University of Technology and FOM Foundation have demonstrated how nanowires of gallium phosphide increases the efficiency of photoelectrochemical conversion of solar energy into fuel. Although it is not as effective as silicon cell connected to the battery, these tests nanowires show increased production of hydrogen (about3%) at the start and increasing up to 10 times compared with solar panels when used at high GaP plane.
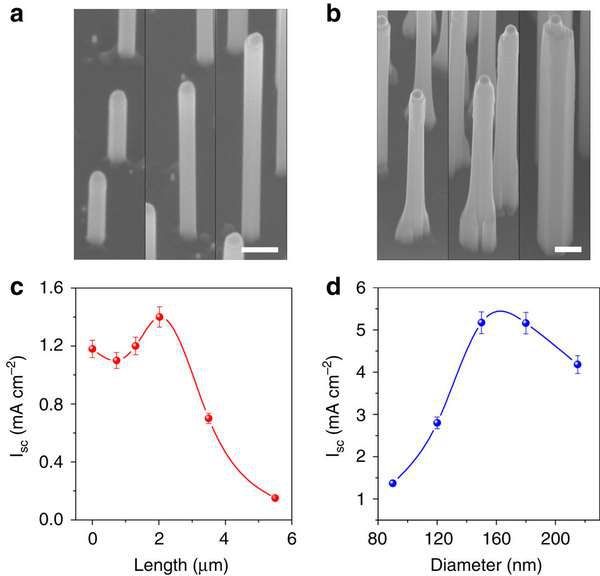
Now steps are being taken to create a perfectly structured nanowires arranged in arrays of 500 nanometers long and 90 nanometers in thickness. This optimization will allow to absorb light waves of different lengths, as well as to reduce the losses due to reflection. This construction requires less GaP and can be coupled to polymers to create flexible devices with minimal cost of material.
Researchers believe that there are still many aspects that need to be refined to improve efficiency. Among the possible ways of considering the addition of an electric field and additional materials, catalysts.